
Rapid Medical Diagnostic Kits Market Share, Size, Trends, Industry Analysis Report, By Product [Professional and Over-The-Counter (OTC)]; By Technology (Agglutination, Solid Phase, Lateral Flow, and Other Technologies); By Application; By End Use; By Regi
- Published Date:Dec-2020
- Pages: 101
- Format: PDF
- Report ID: PM1741
- Base Year: 2019
- Historical Data: 2016-2018
Report Summary
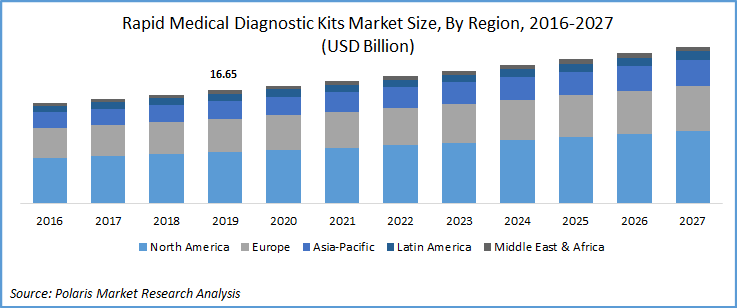
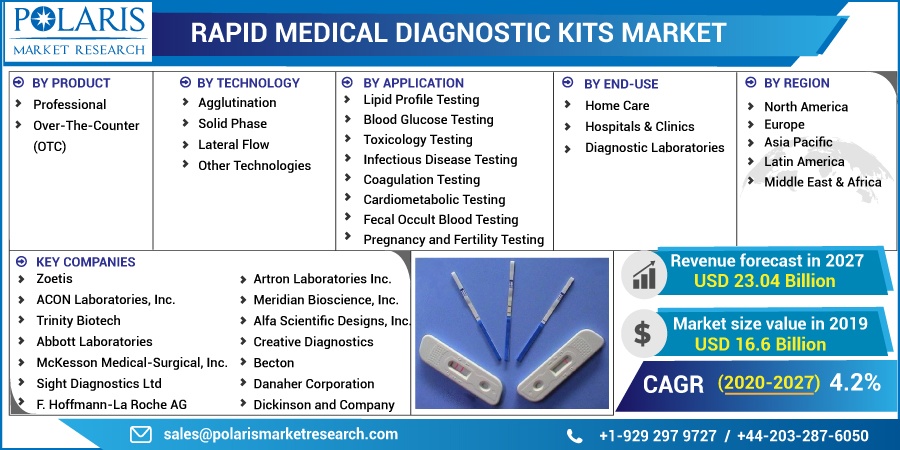
The global rapid medical diagnostic kits market was valued at USD 16.6 billion in 2019 and is expected to grow at a CAGR of 4.2% during 2020-2027. The rising incidences of chronic diseases such as diabetes, cancer, and infectious diseases among the population are expected to drive demand. Increasing demand for point-of-care diagnostics owing to rising health awareness has led to market growth.
Rapid diagnostic test kits are consisting of necessary medical equipment, tools, and devices that are used in emergency situations and are easy to use and can perform quickly. Rapid diagnostic test kits are disposable assays, which is membrane-based and inexpensive and offer rapid treatment to an individual. The product can provide necessary and emergency medical care anywhere to get instant treatment in critical medical condition.
Know more about this report: request for sample pages
Industry Dynamics
Growth Drivers
Rapid medical diagnostic kits are beneficial in managing emergency medical situations. Rising incidences of infectious diseases, diabetes, cancer among individuals, and the outbreak of the COVID-19 has led to the demand for rapid medical diagnostic kits across the globe. For instance, in February 2018, a developer of medical diagnostic tests, Meridian Bioscience announced a launch of its new rapid test i.e., TruQuick, which is designed for the diagnosis of cancer, sexually transmitted, cardiac, infectious respiratory, gastrointestinal, and tropical diseases.
 Know more about this report: request for sample pages
Know more about this report: request for sample pages
The rising awareness for the rapid treatment of the diseases in order to prevent the risk factor with the patient’s life has observed to turnaround the growth of the global market. The ease of usability of the rapid medical diagnostic kits at any place is accounted to secure the major share of the growth of the global market. The outbreak of the COVID-19 has led to appear the need for modern techniques and applications to ensure the point-of-care of the patients on personalized healthcare delivery and consequently enriched the growth of the global rapid medical diagnostic kits market. For instance, in March 2020, Mesa Biotech, Inc., announced to receive the contract of USD 561,000 for the development of rapid medical diagnostic kits to diagnose COVID-19 in individuals, from the U.S. Department of Health and Human Services.
Rapid Medical Diagnostic Kits Market Report Scope
The market is primarily segmented on the basis of product, technology, application, end-use, and geographic region.
|
By Product |
By Technology |
By Application |
By End-Use |
By Regions |
|
|
|
|
|
Know more about this report: request for sample pages
Insight by Product
On the basis of product, the market is divided into professional and over-the-counter (OTC) kits. Professional kits consist of lab tests that are performed in diagnostic & research laboratories and hospitals. Professional laboratory tests are majorly performed with higher test specificity and sensitivity and offer an effective diagnosis of the disease. The professional test kits act as the gold standard in the research applications due to the efficacy and safety of the kits.
The over-the-counter segment has been accounted for witnessing the potential of maximum revenue share in the global market. The OTC kits are mostly utilized in offices, schools, and home care settings, offering a cost-effective and easy alternative to lab testing. Over-the-Counter kits are used as rapid medical diagnostic kits which are easily available in the market and provide instant and quick diagnosis of disease.
Insight by Technology
On focusing on technology, the lateral flow segment accounted for the maximum share of the global market. The dominance of the global market is attributed to the benefits of concerning technology, such as portability and cost-effectiveness. The ease and comfort of using this technology have uplifted the demand for the lateral flow segment in the global market. Based on technology, the market is divided into agglutination, solid phase, lateral flow, and other technologies. Furthermore, pregnancy and malaria tests at home by individuals has escalated the growth of the segments in the global market. Moreover, a rise in the initiatives by several market leaders to develop the novel lateral flow tests for the diagnosis of COVID-19 is expected to support technology segment growth over the forecast period.
Insight by Application
On the basis of application, the global market is segmented into lipid profile, blood glucose, toxicology, infectious disease, coagulation, cardio-metabolic, fecal occult blood, and pregnancy and fertility testing. The blood glucose testing segment is projected to hold the major revenue share of the global market due to the increasing incidences of diabetes in patients. The infectious disease testing segment accounted to witness the lucrative growth owing to the outbreak of COVID-19 and infectious diseases. The growing awareness of an individual for rapid treatment is expected to drive the demand for application segments and subsequently surge the global market growth.
Insight by End-Use
Based on end-use, the global market is prominently divided into home care, hospitals & clinics, and diagnostic laboratories. The hospitals & clinics segment is predicted to witness the largest share of the global market. The rise in the dominance of the hospitals & clinics segment is registered due to the growth of primary care settings which are used in hospitals for the treatment and diagnosis of all diseases. Furthermore, the home care settings segment is also anticipated to register the significant growth of the global market owing to the rising utilization of point-of-care testing services.
Geographic Overview
Geographically, North America anticipates holding the dominant share of the rapid medical diagnostic kits market owing to the high incidences of diabetes and other diseases among the population in the region. The rise in the awareness of the healthcare industry and the constantly increasing needs of the patients leads to augment the growth of the rapid medical diagnostic kits market in the North America region. For instance, in June 2019, Abbott introduced a point-of-care HBA1C test i.e., “Afinion HbA1c Dx” to diagnose diabetic patients.
Europe's market growth of the rapid medical diagnostic kits can be determined by the rising initiatives by the key players such as new launch, provide a new platform or system, etc. Spain, Germany, France, the UK, and Italy are the major markets in Europe. For instance, in May 2019, a manufacturer of point-of-care diagnostics, ACON Laboratories, Inc., launched the On-Call Sure Platform in Latin America and Europe. The platform is specially designed to provide the offerings of professional testing kits.
Additionally, the growth of the Asia Pacific market is expected to witness significant growth. The key players are launching new medical testing kits to deliver the most extensive services of the rapid diagnosis and further leads to couple the growth of the rapid medical diagnostic kits market in the region. In March 2020, Biolidics, a Singapore based company introduced rapid kit 2019-nCoV IgG/IgM for COVID-19. Biolidics has received approval for its recently launched rapid test kit to be used in Singapore from Singapore’s Health Science Authority (HSA) and accredited for the growth of the rapid medical diagnostic kits market in Asia Pacific.
Competitive Insight
The key players of the market are focusing on to surge of their revenue and market share by adopting various strategies such as the new launch of the app, agreements, expansions, partnerships, joint ventures, mergers & acquisitions, and collaboration to enhance their footprints in the market for long term. Some of the major players operating the global market include Zoetis, ACON Laboratories, Inc.; Trinity Biotech, Abbott Laboratories, McKesson Medical-Surgical, Inc.; Sight Diagnostics Ltd, F. Hoffmann-La Roche AG, Artron Laboratories Inc.; Meridian Bioscience, Inc.; Alfa Scientific Designs, Inc.; Creative Diagnostics, Becton, Danaher Corporation, Dickinson and Company, Bio-Rad Laboratories, Inc.; BTNX, Inc.; Cardinal Health, and bioMérieux SA.
License and Pricing
Purchase Report Sections
- Regional analysis
- Segmentation analysis
- Industry outlook
- Competitive landscape
Connect with experts
Suggested Report
- Craft Soda Market Share, Size, Trends, Industry Analysis Report, 2022 - 2030
- Injection Molded Plastic Market Research Report, Size, Share & Forecast by 2020 - 2027
- Sternal Closure Systems Market Share, Size, Trends, Industry Analysis Report, 2021 - 2028
- Air Traffic Management Market Share, Size, Trends, Industry Analysis Report, 2022 - 2030
- Blockchain Technology in the Energy Sector Market Research Report, Share and Forecast, 2018 – 2026

