
Pharmacovigilance Market Share, Size, Trends, Industry Analysis Report, By Service, By Product Life Cycle, By Type, By Process Flow, By Therapeutic Area, By End-Use, By Region; Segment Forecast, 2022 - 2030
- Published Date:Mar-2022
- Pages: 101
- Format: PDF
- Report ID: PM2355
- Base Year: 2021
- Historical Data: 2018 - 2020
Report Summary
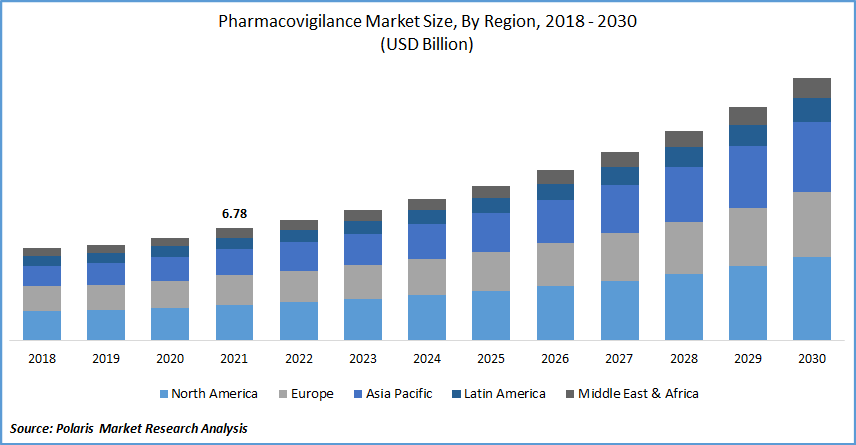
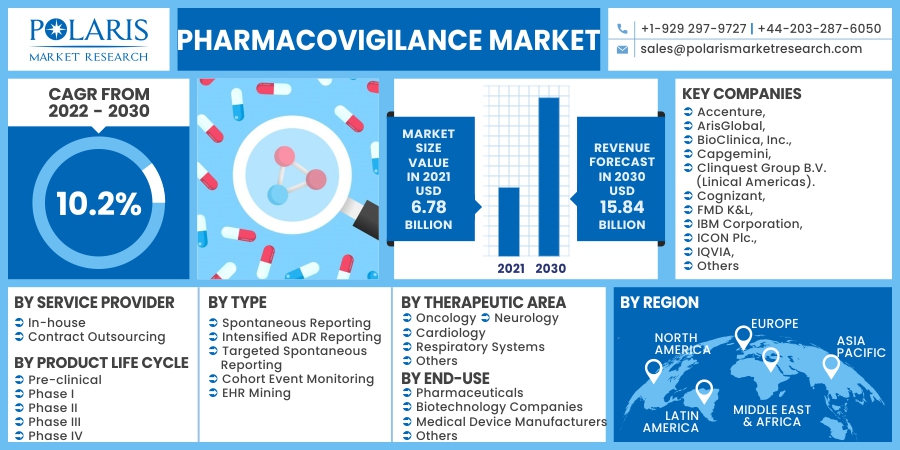
The global pharmacovigilance market was valued at USD 6.78 billion in 2021 and is expected to grow at a CAGR of 10.2% during the forecast period. Pharmacovigilance is the pharmaceutical study of detecting, assessing, comprehending, and preventing harmful consequences, especially long and short negative impacts of medications or therapy.
 Know more about this report: request for sample pages
Know more about this report: request for sample pages
The advent of COVID-19 has created numerous chances for pharmacological firms to develop innovative vaccinations, with multiple therapeutic studies now underway. This element has paved the way for the market, the most miniature crucial stage of every medication production procedure.
Industry Dynamics
Growth Drivers
Strict regulatory rules governing drug delivery, as well as an increase in the number of adverse events caused by medicine, are driving up the need for pharmacovigilance solutions, opening up new opportunities for pharmacovigilance market growth.
Furthermore, rising clinical mistakes, developing clinical infrastructures, and high pharmaceutical manufacturing are lowering deadly ADR incidences and positively impacting the pharmacovigilance industry. Furthermore, governmental requirements for clinical study execution, including post-marketing surveillance, are projected to drive demand for pharmacovigilance solutions over the forecast period.
Know more about this report: request for sample pages
Report Segmentation
The market is primarily segmented based on service provider, product life cycle, type, process flow, therapeutic area, end-use, and region.
|
By Service Provider |
By Product Life Cycle |
By Type |
By Process Flow |
By Therapeutic Area |
By End-Use |
By Region |
|
|
|
|
|
|
|
Know more about this report: request for sample pages
Insight by Service Provider
The contract outsourcing market segment is expected to grow at a considerable pace over the forecast period as a result of improved cost-effectiveness and improved drug risk assessment. The management of vast amounts of information, along with tight legislative requirements, contributes to the growing trend for outsourcing. Furthermore, contract outsourcing is growing in Asia Pacific economies, including China and India, where the expense of outsourcing is significantly cheaper than in western economies, which in turn is boosting the segment growth.
Insight by Type
Spontaneous reporting is expected to hold the largest pharmacovigilance market share over the forecast period due to its widespread use in detecting novel, dangerous, and uncommon ADRs and serving as a practical and cost-effective strategy. The widespread use of monitoring data created using this technology by drug businesses and regulating bodies is also accountable for the section's rapid rise.
Insight by End-Use
Pharmaceuticals are expected to grow at a considerable pace in the global market over the forecast period due to increasing drug innovation by these companies. Furthermore, increasing adoption of outsourcing methods by these companies in order to reduce overhead costs and to increase their resources flexibility. In contrast, the hospital's end-use segment is expected to grow at a significant pace over the forecast period due to increasing hospital admission due to adverse drug reactions.
Geographic Overview
North America dominated the pharmacovigilance market in 2021 and is expected to maintain its dominance during the forecast period due to the presence of major pharmacovigilance companies in the region. Furthermore, increasing manufacturing of drugs, rising investments in biopharmaceutical research & development, along with the accelerating cancer cases in the region are expected to boost the pharmacovigilance demand during the forecast period.
Asia Pacific market is expected to grow at the fastest pace over the forecast period due to the increasing adoption of these services in developing nations like India and China. Increasing population in these countries is also expected to increase the demand for these services over the forecast period.
Competitive Insight
Key players operating in the global pharmacovigilance market include Accenture, ArisGlobal, BioClinica, Inc., Capgemini, Clinquest Group B.V. (Linical Americas). Cognizant, FMD K&L, IBM Corporation, ICON Plc., IQVIA, ITClinical, Laboratory Corporation of America Holdings, Linical Accelovance, PAREXEL International Corporation, TAKE Solutions, United BioSource Corporation, and Wipro Limited.
These companies are involved in strategic partnerships, mergers, and collaborations and invest in new product development to increase their product quality and improve customer safety. For instance, Saama Technologies Inc. released the novel Active Safety Analytics for Pharma solutions in October 2020. ASAP is the first approved pharmacovigilance system to use the FDA's Sentinel Shared Data Format as well as the TreeScan approach for identifying safety indicators.
Pharmacovigilance Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2021 |
USD 6.78 Billion |
|
Revenue forecast in 2030 |
USD 15.84 Billion |
|
CAGR |
10.2% from 2022 - 2030 |
|
Base year |
2021 |
|
Historical data |
2018 - 2020 |
|
Forecast period |
2022 - 2030 |
|
Quantitative units |
Revenue in USD Million/Billion and CAGR from 2022 to 2030 |
|
Segments covered |
By Service Provider, By Product Life Cycle, By Type, By Process Flow, By Therapeutic Area, By End-Use, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
|
Key companies |
Accenture, ArisGlobal, BioClinica, Inc., Capgemini, Clinquest Group B.V. (Linical Americas). Cognizant, FMD K&L, IBM Corporation, ICON Plc., IQVIA, ITClinical, Laboratory Corporation of America Holdings, Linical Accelovance, PAREXEL International Corporation, TAKE Solutions, United BioSource Corporation, and Wipro Limited. |
License and Pricing
Purchase Report Sections
- Regional analysis
- Segmentation analysis
- Industry outlook
- Competitive landscape
Connect with experts
Suggested Report
- Cloud Monitoring Market Share, Size, Trends, Industry Analysis Report, 2022 - 2030
- Household Robots Market Share, Size, Trends, Industry Analysis Report, 2022 - 2030
- Human Milk Oligosaccharides (HMO) Market Share, Size, Trends, Industry Analysis Report, 2022 - 2030
- COVID-19 Vaccine Packaging and Delivery Devices Market Share, Size, Trends, Industry Analysis Report, 2021 - 2028
- Aluminum Composite Panel Market Share, Size, Trends, Industry Analysis Report, 2021 - 2028

